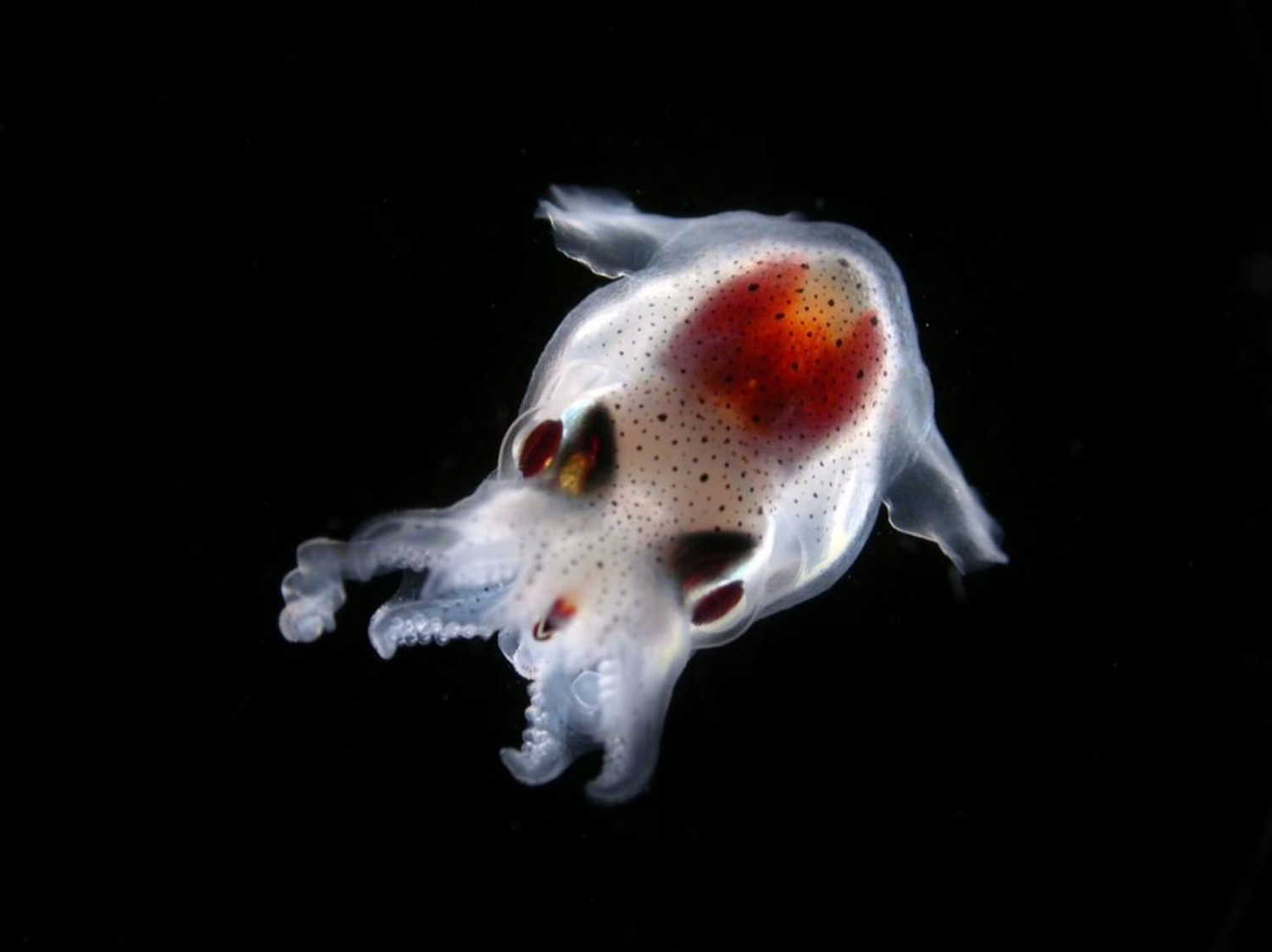
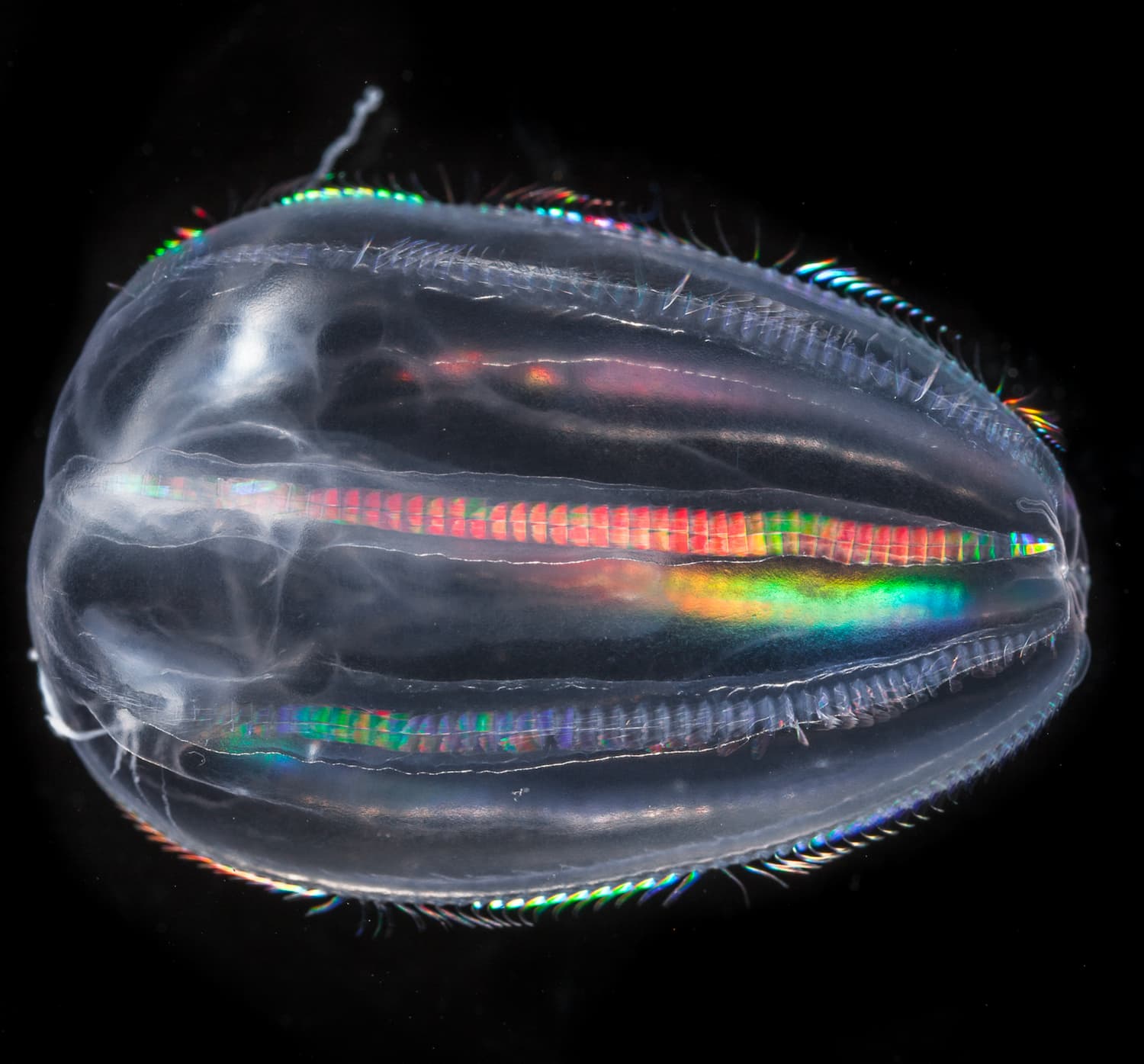
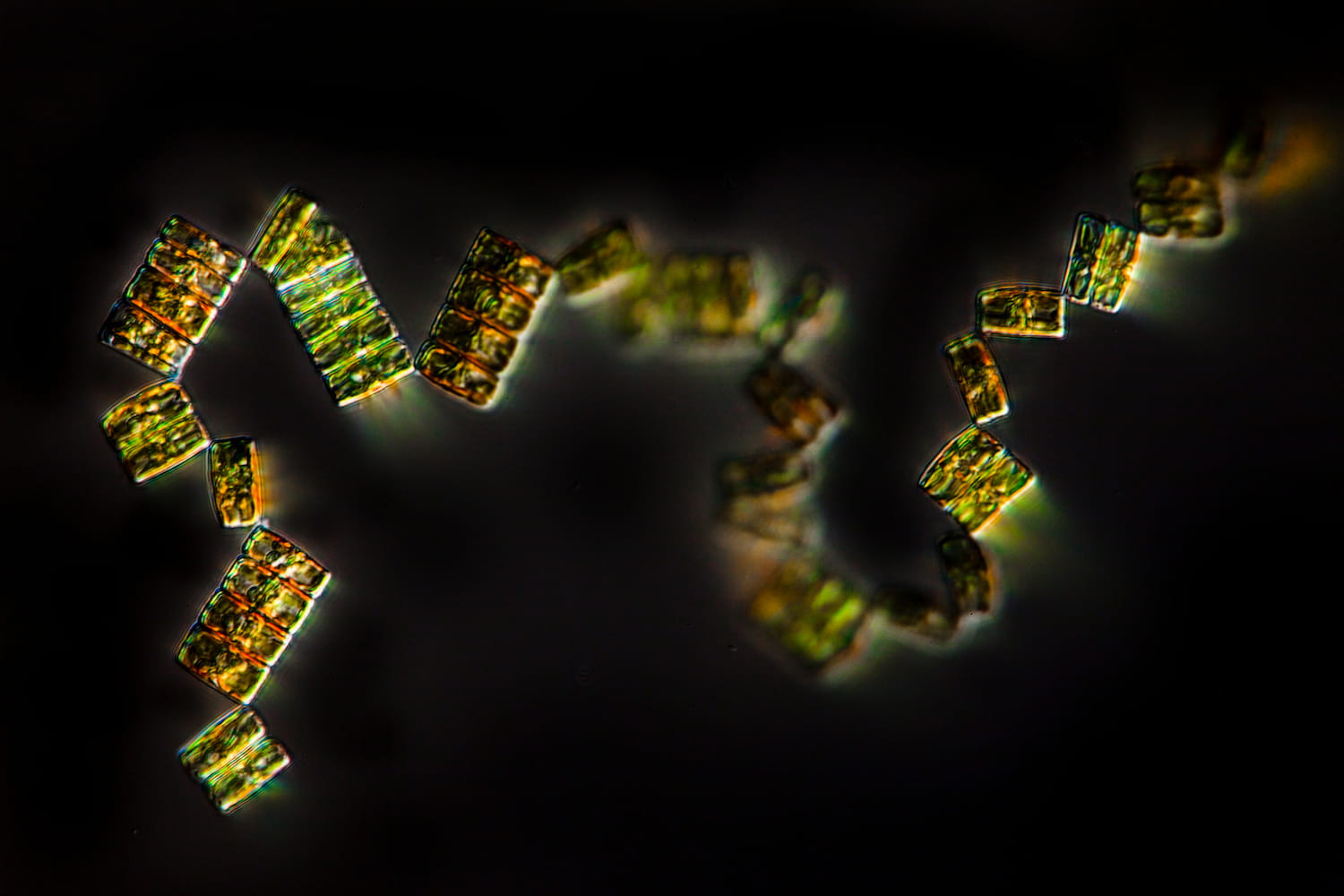An ecosystem in danger
The constant rise in greenhouse gas emissions is causing an increase in global temperatures including the Ocean. This is leading to a domino effect of other damage such as a loss of oxygen or acidification in certain areas. These changes have huge impacts on nearly all marine life forms and on the benefical effects they have each day on the well-being of our societies.
Beyond the problems of submersion affecting populations and infrastructures, the real climate emergency is the risk of losing biodiversity, which only effective sustainable development can enable us to deal with.
- 80 % of marine life is made up of microorganisms
- 50 % of organisms collected by Tara are still unknown
- 30 % of the CO2 emitted each day is captured by the ocean and its biodiversity
- 50 % of the oxygen produced each day is provided by marine microorganisms
Our expeditions
Mission Microbiomes
Current expedition
Better understanding the invisible life of the ocean
Having identified and shared a large number of species of plankton, their genes and their forms with the international scientific community since 2009, this new expedition decided to take an overall approach and treat the oceanic ecosystem as a whole.
To do that, we need to go back to basics, to the first link in this ecosystem: the microbiome.
Tara Pacific
Analyses in progress
Coral reefs stand the test of global warming
The biggest expedition ever carried on coral reefs, the schooner and the teams crossed the Pacific Ocean from East to West to explore thirty reefs from 2016 to 2018. The aim of this mission was to explore the capacities of resistance, adaptation and resilience of coral ecosystems, to discover new life forms as yet hidden and to apply the results for the medical research of the future.
Mais encore …
Invisible life
Our scientific publications
News from Tara Pacific


Eutrophication, an important issue in the Baltic Sea


COP : is a happy ending possible ?
Immerse yourself in our expeditions

Identifying changes to assess their impact for the future
Discover our commitments and research for the Arctic